Chrysosplenetin
 | |
| Names | |
|---|---|
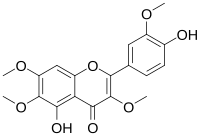
| IUPAC name 4′,5-Dihydroxy-3,3′,6,7-tetramethoxyflavone | |
| Systematic IUPAC name 5-Hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3,6,7-trimethoxy-4H-1-benzopyran-4-one | |
| Other names Chrysosplenetin B 3,6,7,3'-Tetra-methylquercetagetin Quercetagetin 3,6,7,3'-tetramethyl ether | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C19H18O8 |
| Molar mass | 374.345 g·mol−1 |
| Density | 1.448 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
Chrysosplenetin is an O-methylated flavonol. It can be found in the root of Berneuxia thibetica and in Chamomilla recutita.[1]
References
- ^ Miroslav Repčák, Vanda Švehlı́ková, Ján Imrich, Kalevi Pihlaja (1999). "Jaceidin and chrysosplenetin chemotypes of Chamomilla recutita (L.) Rauschert". Biochemical Systematics and Ecology. 27 (7): 727–732. doi:10.1016/S0305-1978(98)00124-0.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- v
- t
- e
Flavonols and their conjugates
| Aglycones |
|
|---|
| Aglycones |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conjugates |
|
| Aglycones |
| ||||
|---|---|---|---|---|---|
| Glycosides |
|
| Aglycones |
|
|---|---|
| Glycosides |
|
| Aglycones |
|---|
| Aglycones | |
|---|---|
| Glycosides |
|
| Glycosides |
|---|
 | This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e









